Contract Manufacturing





- Outline
- FDA Inspections Track Record
- DMFs Filing Record
- Manufacturing Facilities(Ube Chemical Factory)
- Manufacturing Facilities(Yoshitomi Factory)
- Contracting Manufacturing Process for Pharmaceuticals
Outline

UBE offers total solution of APIs & intermediates manufacture.
- UBE supports your projects from development stage to commercial phase.
- UBE's experienced process chemists and engineers establish the optimal manufacturing process.
- UBE also develops and validates test methods upon request.
- UBE manufactures your clinical APIs & intermediates under contract to support your drug development.
- UBE prepares and submits filing documents (e.g. drug master files (DMFs)) upon request.
- UBE provides a stable supply of your compounds after launch utilizing reactors of considerable capacity.
- UBE's manufacturing is controlled based on the current good manufacturing practices (cGMP). UBE had pre-approval inspections (PAI) by the US Food and Drug Administration (FDA). Our latest PAI was in April 2008.
FDA Inspections Track Record
UBE had pre-approval inspections (PAI) by US-FDA, and the customer's products were approved.
| US-FDA | |
|---|---|
| 2002 | Advanced Intermediate (PAI) |
| 2008 | API (PAI) |
UBE also has a variety of inspections and audits by regulatory authorities in Japan as well as a lot of pharmaceutical customers in the US, Europe, and Japan.
DMFs Filing Record
UBE prepares and submits filing documents (e.g. drug master files (DMFs)) upon request.
| US-FDA | Europe | Japan(Manufacturing Approval) | |||
|---|---|---|---|---|---|
| 1998 | API for human drug (IND) | 1998~ | API for animal drug (NDA) | 1993 | API for human drug |
| 1998 | Intermediate for human drug (NDA) | 1995 | API for human drug | ||
| 1999 | Intermediate for animal drug (NDA) | 2000 | API for human drug | ||
| 2000 | API for human drug (IND) | 2003 | API for human drug | ||
| 2004 | API for human drug (IND) | ||||
Manufacturing Facilities (Ube Chemical Factory)

Factory and process R&D are located in the same single site of Ube
city, Yamaguchi prefecture, Japan. Technical transfer from pilot to
commercial is done in the same single site.
The APIs produced in
UBE are all new original drugs for the original pharmaceutical
companies in the US, Europe, and Japan.
No.1 API Plant (cGMP/ API)
| Equipment | Size/Capability | Material | |
|---|---|---|---|
| Reactor | 4,000L(2units) | Glass lined | |
| 3,000L | Glass lined | ||
| 2,000L | Glass lined | ||
| 2,000L | SUS316 | ||
| 1,300L | Glass lined | ||
| Separator,Dryer | Centrifuge | 36inch(2units) | Teflon® lined*1 |
| Centrifuge | 36inch(2units) | SUS316L | |
| Conical dryer | 1,500L | Glass lined | |
| Conical dryer | 1,500L | SUS316 | |
| Distillation column | Sulzer 4,000H(2units) | SUS316 | |

No.2 API Plant Train-1 (cGMP/ API)
| Equipment | Size/Capability | Material | |
|---|---|---|---|
| Reactor | 4,000L(3units) | Glass lined | |
| Separator,Dryer | Centrifuge | 42inch(2units) | Teflon® lined*1 |
| Filter | 1m²(2units) | SUS316 | |
| Filter | 0.8m² | Glass lined | |
| Conical dryer | 3,000L(2units) | Glass lined | |
| Tray dryer | 16m² | SUS316 | |
| Pulverizer | Jet mill | 40kg/h | SUS316 |
| Comil®*3 | 100kg/h | Hastelloy® C-22*2 | |
No.2 API Plant Train-2 (cGMP/ Intermediates)
| Equipment | Size/Capability | Material | |
|---|---|---|---|
| Reactor | 4,000L | Glass lined | |
| 8,000L(4units) | Glass lined | ||
| 8,000L(2units) | SUS316 | ||
| Separator,Dryer | Centrifuge | 48inch | SUS316 |
| Centrifuge | 48inch | Teflon® lined*1 | |
| Filter | 3m² | SUS316 | |
| Filter | 4m² | SUS316 | |
| Filter | 2m² | Hastelloy® C-22*2 | |
| Conical dryer | 2,000L | SUS316 | |
| Conical dryer | 3,000L | SUS316 | |
| Conical dryer | 3,000L | Glass lined | |

No.3 API Plant (cGMP/ Intermediates)
| Equipment | Size/Capability | Material | |
|---|---|---|---|
| Reactor | 8,000L(2units) | Glass lined | |
| 10,000L | SUS316 | ||
| 15,000L(3units) | Glass lined | ||
| Separator,Dryer | Centrifuge | 48inch | Teflon® lined*1 |
| Centrifuge | 48inch | SUS316 | |
| Filter | 4m² | Hastelloy® C-22*2 | |
| Filter | 4m² | SUS316 | |
| Filter | 5m² | SUS316 | |
| Conical dryer | 6,000L | Glass lined | |
| Conical dryer | 6,000L | SUS316 | |

Pilot Plant (cGMP/ API & Intermediates)
Total of 3 lines (Train-A, B, C)
| Equipment | Size/Capability | Material | |
|---|---|---|---|
| Reactor | 2,000L(5units) | Glass lined | |
| 2,000L | Stainless steel | ||
| 1,000L | Glass lined | ||
| 500L(2units) | Glass lined | ||
| 200L | Glass lined | ||
| 50L | Glass lined | ||
| High pressure(~0.7MPa) | 2,600L | Stainless steel | |
| Max blend type | 2,000L | Glass lined | |
| Low temperature(-90~90℃) | 500L | Stainless steel/td> | |
| Separator,Dryer | Centrifuge | 42inch | Teflon® lined*1 |
| Centrifuge | 42inch | Stainless steel | |
| Centrifuge | 30inch | Stainless steel | |
| Filter | 0.3m²(2units) | Glass lined | |
| Filter | 0.2m² | Hastelloy® C-22*2 | |
| Filter | 0.2m² | Glass lined | |
| Filter dryer | 0.2m² | Hastelloy® C-22*2 | |
| Tray dryer | 9.2m² | Stainless steel | |
| Vibrating dryer | 240L | Titanium lined | |
| Conical dryer | 1,000L | Glass lined | |
| Conical dryer | 1,000L | Stainless steel | |
| Pulverizer | Jet mill | 10kg/h | Stainless steel |
| Hammer mill | 10-30kg/h | Stainless steel | |
| Comil®*3 | 40kg/h | Stainless steel | |

*1Teflon® is a registered trademark of Du Pont in Japan and other countries.
*2Hastelloy® is a registered trademark of Haynes International, Inc. in Japan and other countries.
*3Comil® is a registered trademark of Quadro Engineering Corporation in Japan and other countries.
Manufacturing Equipment (Yoshitomi Factory)
We offer the Safe, Stable, Low-cost, and High-quality APIs and intermediates at our cGMP-compliant multipurpose plants. We supply APIs and intermediates under Quality assurance to all steps such as raw material procurement, manufacturing, processing, and shipping.
Commercial plant
| Dangerous reactions | Tetrazolization, diazotization, nitration, hydrogen reduction, Grignard,reaction |
|---|---|
| Others | Amination, alkylation, imidization, iminoethylation, ethylation, esterification, carbamoylation, sulfonation, dehalogenation, thioamination, disulfidization, nitrosylation, Friedel-Crafts reaction, Sandmeyer reaction, hydroboration, halogenation, hydrazine, condensation, methylolation, salt synthesis, hydrolysis, reduction synthesis, optical separation, acid amination, acid chloride, decarboxylation, Kolbe-Schmitt, reaction, etc. |
| Water-reactive reagents | n-BuLi, sec-BuLi, 60% NaH, etc. |
|---|---|
| Fire Services Act Class 5 Dangerous Goods | Organic peroxides, NaN3, hydroxylamine hydrochloride, etc. |
| Other | Phosphorus pentasulfide, inorganic CN compounds, diborane, bromine, etc. |
| Reactor |
0.5m3-20m3 GL, Stainless steel, HC equivalent Anchor blade, Three-blade retreat impeller, Highly-efficient multifunctional mixing impeller, etc. |
|---|---|
| Filter (Centrifuge) |
24-48 inches Stainless steel, HC equivalent, Teflon lining vertical centrifuge (upper exhaust and lower exhaust types) |
| Drying equipment |
1-7m3 Stainless steel, HC equivalent, Teflon lining vertical centrifuge (upper exhaust and lower exhaust types) |
| Mills |
16 Pin mill, cutter mill, hammer mill, jet mill, etc. |
| Clean room |
9 Maintained at Class 100,000 |
| -80 to 180℃ | UTT: liquid nitrogen, brine, chilling water, steam (190℃) |
|---|
| FV approx. 0.99 Mpa |
|---|
Pilot plant
| Same as that of commercial facilities and as per clients' requests |
|---|
| As per clients requests |
|---|
| Reactor |
100-8000 L GL: Total 50 m3 Stainless steel: Total 30 m3 Anchor blade, Three-blade retreat impeller, Highly-efficient multifunctional mixing impeller, Molepaw blade, etc. |
|---|---|
| Filter (Centrifuge) |
24-42 inches Stainless steel, HC equivalent |
| Drying equipment |
200-850 L GL |
| Mills |
4 Pin mill, cutter mill, hammer mill, jet mill |
| Clean room |
1 Maintained at Class 100,000 |
| -80 to 180℃ | UTT: Liquid nitrogen, brine, chilling water, steam (190℃) |
|---|
| FV approx. 0.97 MPa |
|---|
Analytical Equipment
| Chromatography system | High Performance Liquid Chromatography (HPLC), Ultra High Performance Liquid Chromatography (UHPLC), Gas Chromatography (GC), Ion Chromatography (IC) |
|---|---|
| Spectral photometer | Fourier Transform Infrared Spectroscopy (FT-IR), UV-vis Spectrophotometer, Polarimeter, Refractometer, Color difference meter |
| Powder Analysis | X-Ray Powder Diffraction, Particle counter |
| Nuclear Magnetic Resonance | 400MHz-NMR |
| Mass Spectrometer | Inductively Coupled Plasma Mass Spectrometry (ICP/MS), Liquid Chromatography-Mass spectrometry (LC-MS), Gas Chromatography - Mass spectrometry (GC/MS) |
| Thermal Analysis | Differential Scanning Calorimetry (DSC), Thermogravimetry/Differential Thermal Analysis (TG/DTA) |
| Others | pH meter, Melting point apparatus, TOC analyzer, Karl Fischer titrator (volumetric and coulometric), Titrator, Hydrometer, Electrical conductivity meter |
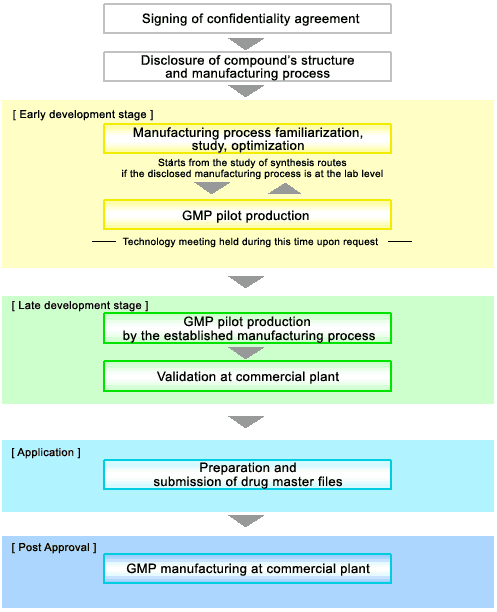
Contracting Manufacturing Process for Pharmaceuticals
Inquiry
Products Inquiry
